Vol. 1, Issue 1 (2013)
Determination of Trace Amount of Cu(II) Using UV-Vis. Spectrophotometric Method.
Author(s): Khokan Chandra Sarker, Md. Rafique Ullaha
Abstract: Trace amount of Copper(II) has determined by spectrophotometric technique using 1-(2-pyridylazo)-2-naphthal (PAN), as a new spectrophotometric reagent which is insoluble in water. PAN reacts in highly acidic solution at pH 2.40 to 2.50 with Cu(II) to give a pink chelate which has an absorption maximum (λmax.) at 550nm. The reaction is instantaneous and absorbance remains stable for over 48 hrs. The average molar absorption co-efficient (ε) was found to be 2.05×104 L mol-1cm-1 and Sandell sensitivity is 3.23×10−4 μg cm−2. Linear calibration graphs were obtained for 0.1-4.0 μg mL-1 of Cu(II) and RSD (%) is 1.16. The stoichiometric composition of the chelate is 1:2 (Cu:PAN). Large excess of over 50 cations, anions, and some common complexing agents (e.g. oxalate, phosphate, tartarate, thio-urea) do not interfere in the determination. The method was successfully used in the determination of Cu(II) in Several Standard Reference Materials as well as in some environmental and industrial waste water. The method has high precision and accuracy.
Related Graphics: Click here for more related graphics
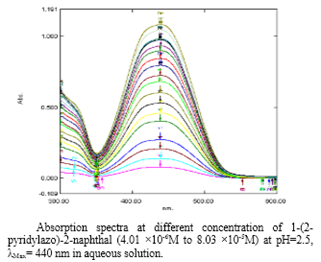
Fig. 1: images/2.1.png
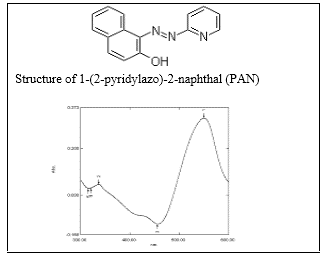
Fig. 2: Absorption spectrum of Cu (II)-PAN against the reagent blank (at pH=2.50, λMax=550 nm) in aqueous solution
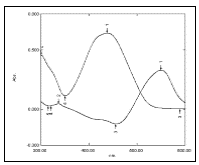
Fig. 3: Absorption spectrum of PAN and Cu (II)-PAN against the reagent blank at pH=2.50 in aqueous solution
Pages: 05-14 | 3919 Views 293 Downloads
download (10295KB)
How to cite this article:
Khokan Chandra Sarker, Md. Rafique Ullaha. Determination of Trace Amount of Cu(II) Using UV-Vis. Spectrophotometric Method.. Int J Chem Stud 2013;1(1):05-14.






