Vol. 2, Issue 5 (2015)
Friedel-Crafts alkylation and acylation of aromatic compounds under solvent free conditions using solid acid catalysts
Author(s): Shrinivas V. Ghodke and Uma V. Chudasama
Abstract: Solid acid catalysts, M(IV) Phosphotungstates [M(IV)PWs], mixed materials of the class of TMBA (Tetravalent Metal Bianionic Acid) salts and 12-Tungstophosphoric acid (12-TPA) supported onto M(IV) Oxides [M(IV) = Zr, Ti, Sn] have been synthesized and their utility towards Friedel–Crafts alkylation and acylation reactions has been explored and compared. The catalysts have been characterized for elemental analysis by ICP-AES, TGA, FTIR, SEM, EDX, XRD, surface area (BET) and surface acidity (NH3-TPD). Friedel-Crafts alkylation and acylation have been studied as model reactions under solvent free conditions wherein acetyl chloride is used for acylation of anisole/veratrole and benzyl chloride is used for alkylation of toluene. Reaction conditions have been optimized by varying parameters such as reaction time, reaction temperature, catalyst amount and mole ratio of the reagents. Highlighting features are selectivity of products under solvent free conditions and regeneration/reuse of catalysts without much loss in % yields.
Related Graphics: Click here for more related graphics
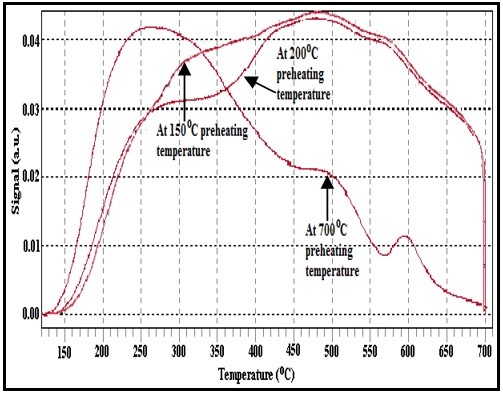
Fig. 1: NH3-TPD patterns of SnPW.
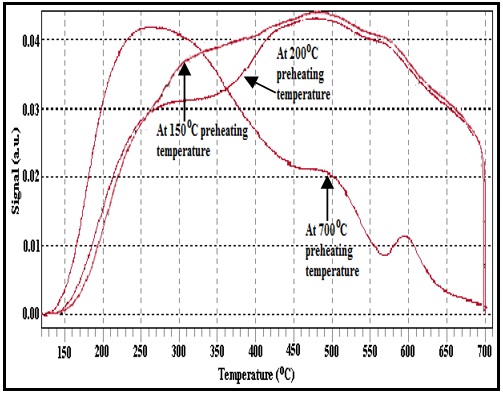
Fig. 2: NH3-TPD patterns of TiPW
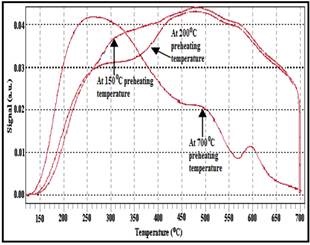
Fig. 3: NH3-TPD patterns of SnPW
Pages: 27-34 | 2851 Views 172 Downloads
download (8331KB)
How to cite this article:
Shrinivas V. Ghodke, Uma V. Chudasama. Friedel-Crafts alkylation and acylation of aromatic compounds under solvent free conditions using solid acid catalysts. Int J Chem Stud 2015;2(5):27-34.






