Vol. 1, Issue 4 (2013)
A newer validated and stability indicating HPLC method for the estimation of Atenolol and Hydrochlorothiazide in bulk drug and dosage form
Author(s): Vilas Chaudhari, Syed Hussian, Milind Ubale
Abstract: A rapid high performance liquid chromatographic method has been developed and validated for the estimation of atenolol and Hydrochlorothiazide simultaneously in combined dosage form. A RP stainless steel ODS C-18 column having dimensions of 4.6×250 mm and particle size of 5 μm in isocratic mode, with mobile phase containing a mixture of phosphate buffer (0.03 M), pH 3.5 adjusted with orthophosphoric acid and acetonitrile: in the proportion (50:50 v/v) .The mobile phase was pumped at a flow rate of 1.0 ml/min and the eluent were monitored at 226 nm. The developed method was validated with respect to linearity, accuracy, recovery, precision and solution stability studies prove the stability indicating stability of the method.
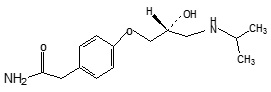
Fig. 1: Chemical Structure of Atenolol
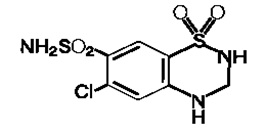
Fig. 2: Molecular formula-C7 H8ClN3O4S2
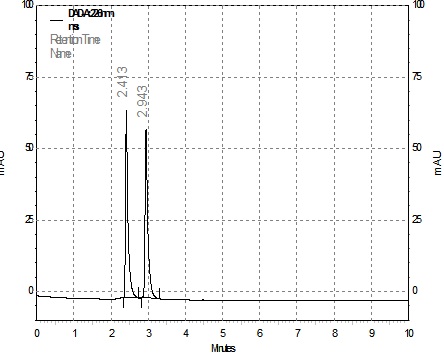
Fig. 3: Chromatogram of Specificity. A Chromatogram of the Atenolol and Hydrochlorothiazide in diluted standard
Pages: 93-101 | 1785 Views 58 Downloads
download (2450KB)
How to cite this article:
Vilas Chaudhari, Syed Hussian, Milind Ubale. A newer validated and stability indicating HPLC method for the estimation of Atenolol and Hydrochlorothiazide in bulk drug and dosage form. Int J Chem Stud 2013;1(4):93-101.






