Vol. 1, Issue 3 (2013)
Kinetic studies of oxidation of Ethambutol in basic media
Author(s): Sandipsingh Gour, R.P. Phase,B R Agarwal, Mazahar Farooqui
Abstract: The oxidation of pyrazinamide in acidic media is carried out using potassium permanganate as a oxiding agent. The reaction was monitored using UV-Visible spectrophotometer at 525 nm. It was found to be zero order with respect to oxidant,, fractional order with respect to hydrogen ion concentration and first order with respect to substrate. The thermodynamic parameters(were determinied . The average (ΔG#) was found to be 86.508 KJ/mol. The values ΔS# was found to be -0.1354 KJ/mole and energy of activation was found to be 46.7709 KJ/mole. A suitable mechanism is proposed based on the experimental conditions.
Related Graphics: Click here for more related graphics
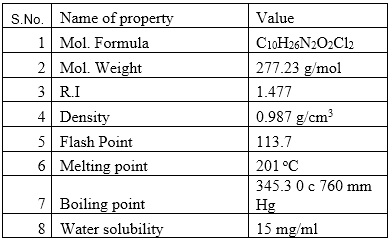
Fig. 1: Physical properties of Ethambutol
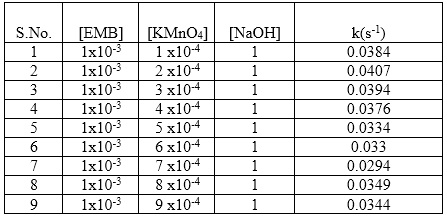
Fig. 2: First order rate constant
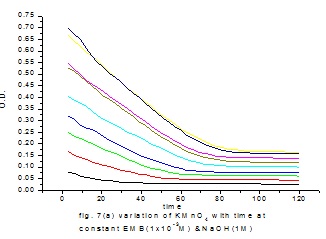
Fig. 3: images/16.3.jpg
Pages: 107-113 | 1604 Views 53 Downloads
download (5640KB)
How to cite this article:
Sandipsingh Gour, R.P. Phase,B R Agarwal, Mazahar Farooqui. Kinetic studies of oxidation of Ethambutol in basic media. Int J Chem Stud 2013;1(3):107-113.