Vol. 1, Issue 3 (2013)
A Newer Validated and Stability Indicating GC Method for the Estimation of Lindane in Formulation
Author(s): V. Sreeram*, M.V. Basaveswara Rao, A.V.D. Nagendrakumar
Abstract: A simple, selective, linear, precise and accurate GC Method was developed and validated for rapid assay of Lindane in Formulation. Isocratic elution at a flow rate of 1.0ml/min was employed on DB 1,30 m × 0.53 mm Capillary column at ambient temperature250 °C. Injection Volume was found to be 2 µl. The mobile phase consisted of Acetone: Chloroform 80:20 v/v. The UV detection wavelength was 240nm and 2µl sample was injected. The retention time for Lindane was 6.2 min. A linear regression curve was constructed, and the correlation coefficients (R2) and assessment values calculated The percentage RSD was found to be 1.0%.The Accuracy of method ranges between 97.0 – 102%. The method was validated as per the ICH guidelines. The method was successfully applied for routine quality control analysis of pharmaceutical formulation.
Related Graphics: Click here for more related graphics
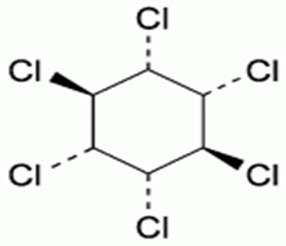
Fig. 1: Structure of Lindane
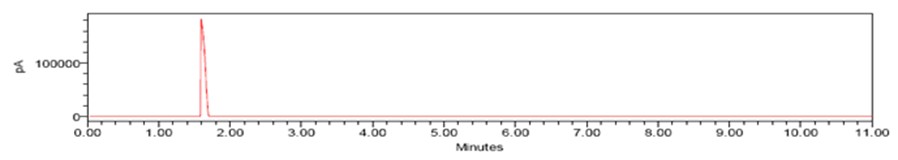
Fig. 2: Chromatogram 1
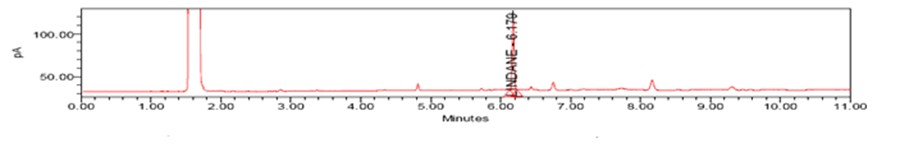
Fig. 3: Chromatogram 2
Pages: 66-72 | 1782 Views 75 Downloads
download (6519KB)
How to cite this article:
V. Sreeram*, M.V. Basaveswara Rao, A.V.D. Nagendrakumar. A Newer Validated and Stability Indicating GC Method for the Estimation of Lindane in Formulation. Int J Chem Stud 2013;1(3):66-72.






