Vol. 1, Issue 3 (2013)
Synthesis, Characterization and Density Functional Theory Studies of 3-Chlorochromones
Author(s): A. B. Sawant*, R. S. Nirwan
Abstract: 3-Chlorochromones like 3-chloro-2-(4-methoxyphenyl)-4H-chromen-4-one, 3,6-dichloro-2-(4-chlorophenyl)-4H-chromen-4-one, 6-bromo-3-chloro-2-(4-chlorophenyl)-4H-chromen-4-one, 3,6-dichloro-2-(4-methoxyphenyl)-4H-chromen-4-one and 6-bromo-3-chloro-2-(4-methoxyphenyl)-4H-chromen-4-one have been synthesized and characterized by spectroscopic methods. The density functional theory (DFT) calculations of these molecules were at B3LYP/6-311++G(d,p) level. Optimized geometries, 1H NMR and harmonic fundamental vibrational frequencies were evaluated. The vibrational frequencies and 1H NMR determined experimentally were compared with those obtained theoretically. Thermodynamic properties like entropy, heat capacity, zero point energy, dipole moment, bond length, bond angels, atomic charges and HOMO-LUMO have also been recorded. In addition to this electronic spectra of these molecules are calculated by using TD-DFT method and compared with experimental spectrum.
Related Graphics: Click here for more related graphics
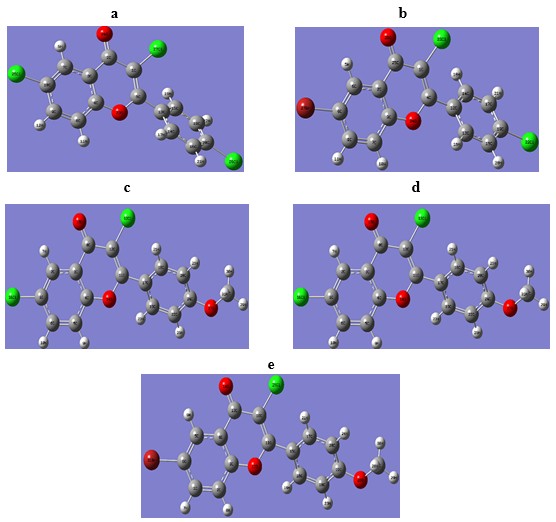
Fig. 1: DFT Optimized geometries of compounds using B3LYP/ 6-311++G(d,p) basis set.
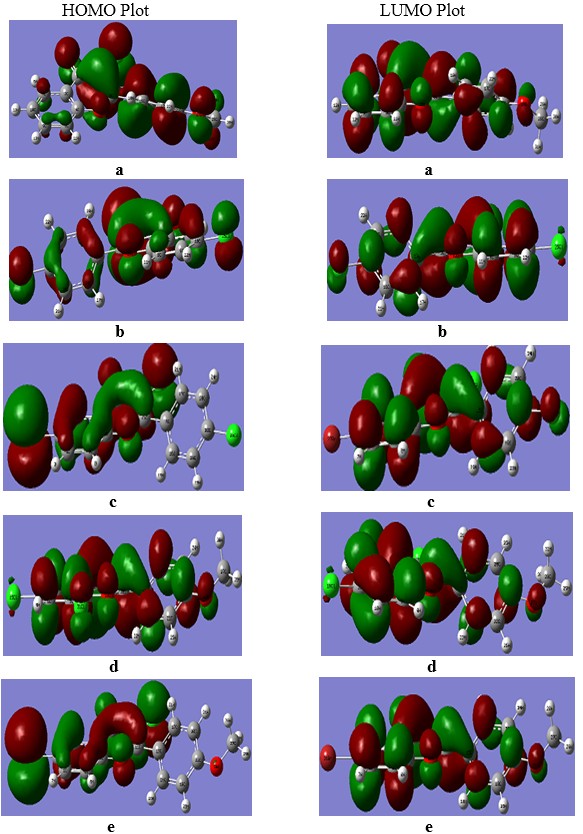
Fig. 2: Frontier Molecular orbitals of compound a, b, c, d, and e.
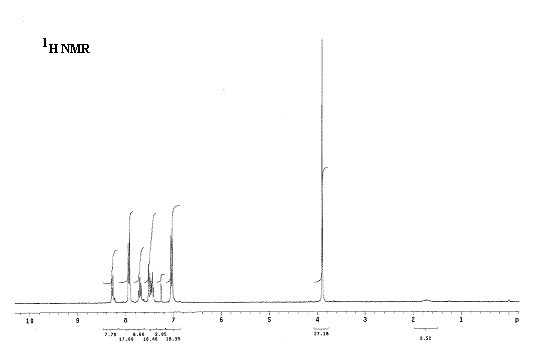
Fig. 3: Experimental 1H NMR spectrum of compound a.
Pages: 47-65 | 1755 Views 73 Downloads
download (7528KB)
How to cite this article:
A. B. Sawant*, R. S. Nirwan. Synthesis, Characterization and Density Functional Theory Studies of 3-Chlorochromones. Int J Chem Stud 2013;1(3):47-65.






