Vol. 1, Issue 2 (2013)
Defluoridation of Water by Precipitation with Calcium Sulfate and Calcium Chloride
Author(s): Djamel Atia, and Bebba Ahmed Abdelhafidh*
Abstract: El-Oued is known for some diseases caused by fluoride concentration in drinkable water. To reduce it, we have chosen a sample with the highest content of fluoride among many sources in order to precipitate it with CaCl2 and CaSO4. In order to get better reduction yield of fluoride, a study has been done on the influencing parameters (concentration, pH, temperature) to choose the best conditions. The remove of fluoride is favorable at low concentration of Ca(OH)2, at room temperature and normal acidity.
Related Graphics: Click here for more related graphics
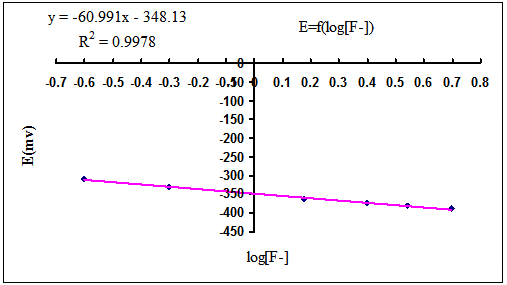
Fig. 1: The witness graph for fluoride
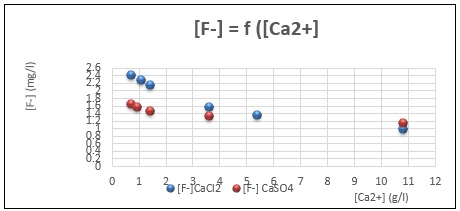
Fig. 2: variation of residual fluoride against added calcium concentration
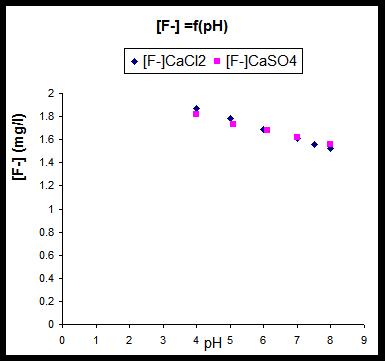
Fig. 3: variation of residual fluoride against pH
Pages: 39-44 | 1788 Views 72 Downloads
download (6897KB)
How to cite this article:
Djamel Atia,, Bebba Ahmed Abdelhafidh*. Defluoridation of Water by Precipitation with Calcium Sulfate and Calcium Chloride. Int J Chem Stud 2013;1(2):39-44.






