Vol. 1, Issue 1 (2013)
A Validated and Stability Indicating Ultra High Pressure Liquid Chromatographic Method for Folic Acid in Pharmaceutical Preparation.
Author(s): V. Sreeram*, M.V. Basaveswara Rao, Srinivasa Rao Karumuri
Abstract: A simple, selective, linear, precise, and accurate ultra-high performance liquid chromatography method was developed, optimised and validated for the quantification of synthetic folic acid (FA) in pharmaceutical dosage form. Isocratic elution at a flow rate of 0.4 mL/min was employed on C8 1.7 µm (2.1 mm x 100 mm) or equivalent column at ambient temperature. The mobile phase consists of Acetonitrile :0.005 M 1–Hexane Sulfonic Acid Salt. PH 2.5 with Phosphoric acid in the ratio of 10:90 v/v. The UV detection wavelength was 210 nm, and5μL sample was injected. The Flow rate was found to be 0.4 ml/min The retention time for folic acid was ± 2.0 min. The percent RSD for accuracy of the method was found to be 0.2%. The correlation coefficient (R2) for Folic Acid is 1.000. The average percent Recovery is varying from 104.5 – 96.9. The method for the Dissolution of Folic Acid 5 mg Tablets complies with the requirements for Specificity, System suitability, Linearity, Accuracy and Method precision across the range of 25 % to 125 %. The method is therefore acceptable as valid and stability indicating. The method was validated as per the guidelines. The method can be successfully applied for Folic acid in the rapid and reliable determination of folic acid in pharmaceutical dosage form.
Related Graphics: Click here for more related graphics
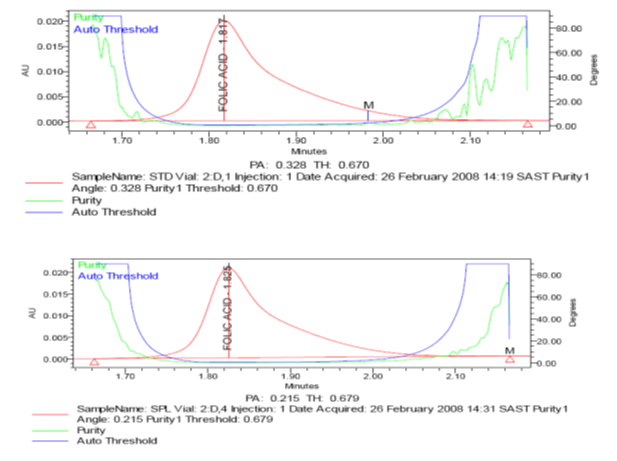
Fig. 1: images/3.1.pn
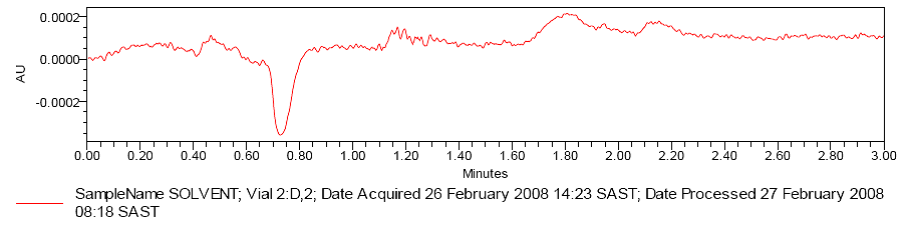
Fig. 2: Chromatogram 1
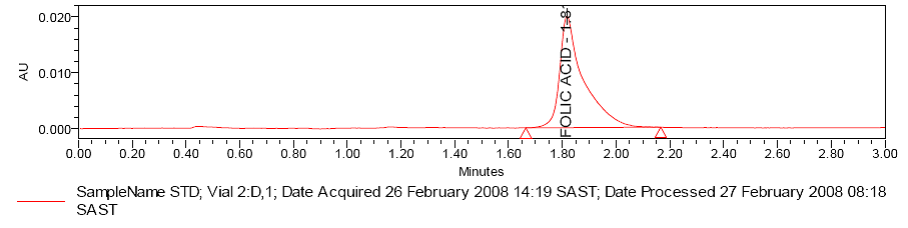
Fig. 3: Chromatogram 2
Pages: 17-27 | 2777 Views 81 Downloads
download (12535KB)
How to cite this article:
V. Sreeram*, M.V. Basaveswara Rao, Srinivasa Rao Karumuri. A Validated and Stability Indicating Ultra High Pressure Liquid Chromatographic Method for Folic Acid in Pharmaceutical Preparation.. Int J Chem Stud 2013;1(1):17-27.






