Vol. 1, Issue 5 (2014)
Studies on metal ligand stability constant of allopurinol and succinic acid with some metal ions in aqueos media
Author(s): Jamilkhan Pathan, D M Janrao, B R Agarwal, Mazahar Farooqui
Abstract: The PH-Metric stability constant of binary & ternary complexes of allopurinol with bivalent metal & Succinic acid in aqueous solution has been determined. The ionic strength was kept constant by sodium nitrate. The stability constant of ternary complexes have been quantitatively compared with those of corresponding binary complexes in terms of the parameter ΔlogK.
Related Graphics: Click here for more related graphics
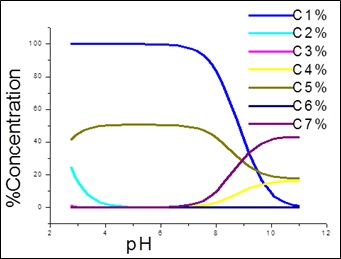
Fig. 1: Species distribution curves for APN +SA +Fe(III)
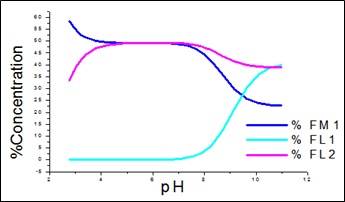
Fig. 2: Percentage conc. of free metal and free ligands for APN +SA +Fe(III).
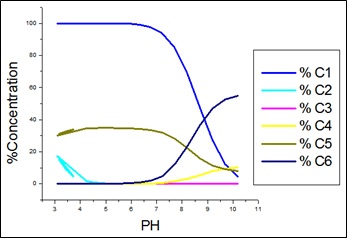
Fig. 3: Species distribution curves for APN +SA +Co(II).
Pages: 36-41 | 1765 Views 91 Downloads
download (6399KB)
How to cite this article:
Jamilkhan Pathan, D M Janrao, B R Agarwal, Mazahar Farooqui. Studies on metal ligand stability constant of allopurinol and succinic acid with some metal ions in aqueos media. Int J Chem Stud 2014;1(5):36-41.






