Vol. 1, Issue 3 (2013)
Thermodynamics of the Formation of Trivalent Lanthanide Complexes Carrying Adenosine Drug in Mixed Solvent Media
Author(s): Shailendra singh Thakur *, Sahebrao Naikwade and Mazahar Farooqui
Abstract: The stability constant of Adenosine drug with trivalent lanthanide metal ions La3+, Ce3+, Nd3+, Sm3+, Gd3+, Tb3+ and Dy3+ using a pH metric titration technique in 20% (v/v) ethanol-water mixture at three different temperatures (25 °C, 35 °C and 45 °C) and at an ionic strength of 0.1M NaClO4 were studied. The method of Calvin and Bjerrum as adopted by Irving and Rossotti has been employed to determine metal-ligand stability constant (logK) values. The trend in the formation constants follows the order: La3+< Ce3+< Nd3+< Sm3+> Gd3+< Tb3+< Dy3+ and it shows a break at gadolinium. The thermodynamic parameters such as Gibb’s free energy change (∆G), entropy change (∆S) and enthalpy change (∆H) associated with the complexation reactions were also calculated. The formation of metal complexes was found to be spontaneous and exothermic in nature.
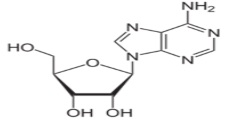
Fig. 1: images/34.2.jpg
Pages: 88-92 | 1563 Views 55 Downloads
download (4581KB)
How to cite this article:
Shailendra singh Thakur *, Sahebrao Naikwade, Mazahar Farooqui. Thermodynamics of the Formation of Trivalent Lanthanide Complexes Carrying Adenosine Drug in Mixed Solvent Media. Int J Chem Stud 2013;1(3):88-92.






